Weekly reads 25/1/27
This marks the first week of my attempt to publicly summarize the papers I’ve read. Typically, I aim to read at least one paper per day during my commute. However, this week, I’m taking the weekend to recharge after submitting a paper.
I’m still figuring out the best way to approach these summaries, so expect some adjustments in future posts. For now the concept is heavily borrowed from the fantastic Paired Ends substack.
Preprints/articles that i managed to read this week
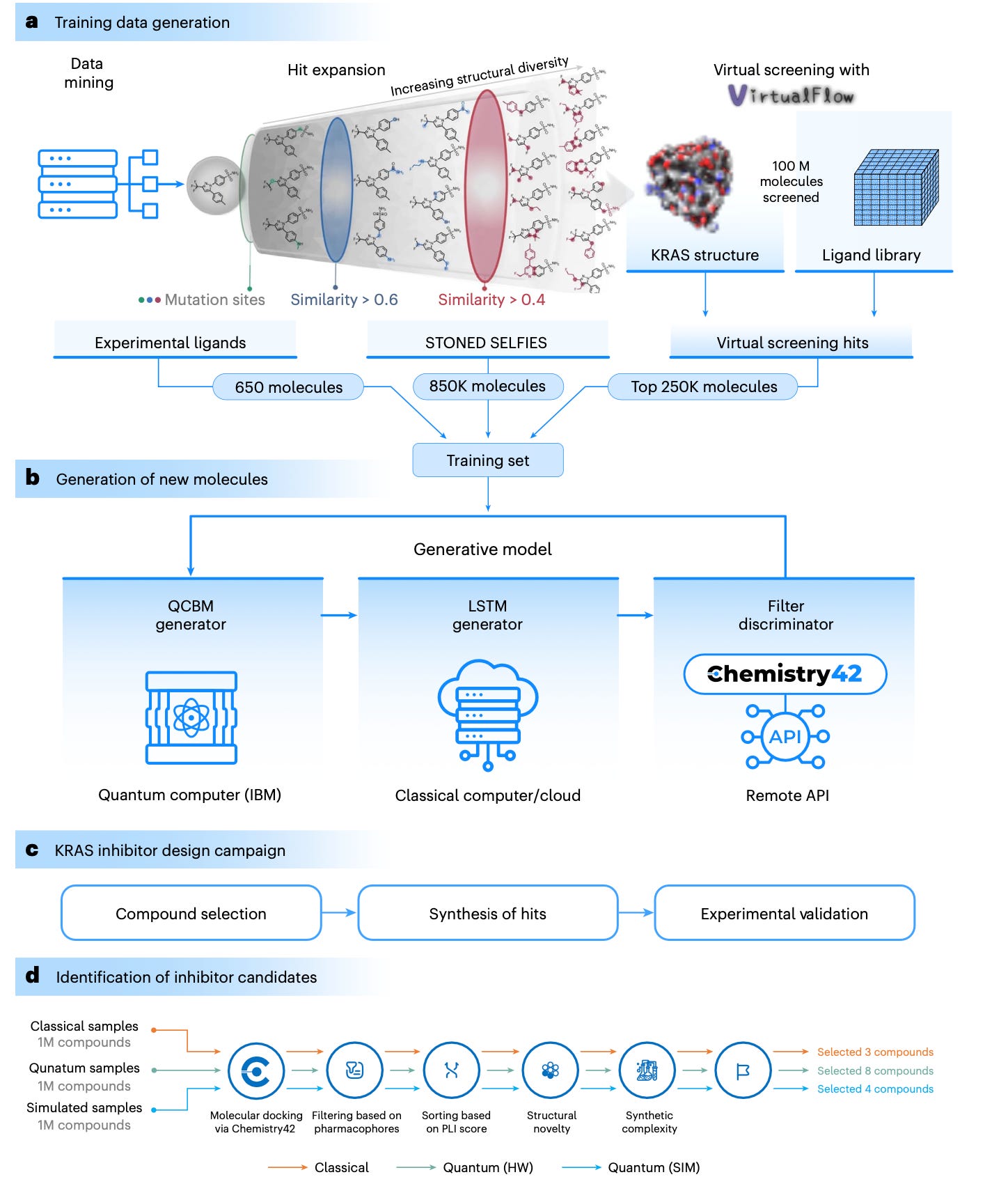
Quantum-computing-enhanced algorithm unveils potential KRAS inhibitors
Ghazi Vakili, M., Gorgulla, C., Snider, J. et al. Quantum-computing-enhanced algorithm unveils potential KRAS inhibitors. Nat Biotechnol (2025). https://doi.org/10.1038/s41587-024-02526-3
The paper in one sentence
A hybrid quantum-classical generative model was developed to design and experimentally validate small-molecule KRAS inhibitors, demonstrating the potential of quantum computing in drug discovery.
Summary
The paper presents a hybrid quantum-classical generative model for designing small-molecule KRAS inhibitors, a challenging target in cancer therapy. The model combines quantum circuit Born machines (QCBMs) with classical long short-term memory (LSTM) networks to generate novel molecules. The authors trained the model on a dataset of over 1 million molecules, including experimentally validated KRAS inhibitors and virtual screening hits. From the generated molecules, 15 compounds were synthesized and experimentally validated, with two (ISM061-018-2 and ISM061-022) showing promising binding affinity and biological activity against KRAS mutants. The study demonstrates the potential of quantum computing to enhance drug discovery by efficiently exploring complex chemical spaces and generating experimentally validated hits.
Personal highlights
Hybrid Quantum-Classical Model: The model integrates quantum circuit Born machines (QCBMs) with classical LSTM networks to generate novel molecules.
Training Data Expansion: The dataset was expanded using the STONED-SELFIES algorithm and virtual screening of 100 million molecules from the Enamine REAL library.
Quantum Computing: The QCBM leverages quantum effects like superposition and entanglement to explore complex chemical spaces more efficiently than classical models.
Experimental Validation: The top 15 generated compounds were synthesized and tested using surface plasmon resonance (SPR) and cell-based assays, with two compounds showing strong binding affinity and biological activity.
Why should we care?
This work highlights the transformative potential of quantum computing in biomedical sciences, reducing the time and resources required for drug discovery. By tackling the notoriously challenging KRAS protein, it offers hope for breakthroughs in cancer treatment and a paradigm shift in how computational methods can address drug design challenges. Also quantum computing just sounds awesome :)
Immune evasion through mitochondrial transfer in the tumour microenvironment
Ikeda, H., Kawase, K., Nishi, T., et al. (2025). Immune evasion through mitochondrial transfer in the tumour microenvironment. Nature. DOI:10.1038/s41586-024-08439-0
The paper in one sentence
This study uncovers a novel immune evasion mechanism where cancer cells transfer mitochondria with mutated mitochondrial DNA to tumor-infiltrating lymphocytes, impairing their antitumor functions and promoting immune resistance.
Summary
The research explores how cancer cells evade immune detection by transferring mitochondria containing mutated mitochondrial DNA (mtDNA) to tumor-infiltrating lymphocytes (TILs). This process leads to mitochondrial dysfunction, metabolic abnormalities, and cellular senescence in TILs, reducing their antitumor immune activity. The study further reveals that this phenomenon contributes to poor responses to immune checkpoint inhibitors in cancers like melanoma and non-small-cell lung cancer. By identifying the molecular and cellular mechanisms underpinning this mitochondrial transfer, including the role of mitophagy-resistant mitochondria and associated inhibitory molecules, the findings open up new avenues for enhancing immunotherapy effectiveness.
Personal highlights
Mitochondrial Transfer and Mutation Sharing: Cancer cells transfer mitochondria with mtDNA mutations to TILs, leading to metabolic reprogramming and dysfunction.
Mitophagy Resistance: Mitochondria from cancer cells resist degradation in TILs due to the presence of mitophagy-inhibitory molecules like USP30.
Functional Impairments in TILs: mtDNA-mutated TILs exhibit senescence, reduced memory formation, and diminished effector functions, compromising antitumor immunity.
Clinical Relevance: The presence of mtDNA mutations in tumors correlates with poor prognoses for patients treated with immune checkpoint inhibitors.
Why should we care?
This work highlights a critical, previously unrecognized mechanism of immune evasion by cancer, linking mitochondrial biology to immunotherapy resistance. It underscores the importance of mitochondrial dynamics in cancer biology and offers potential therapeutic strategies to counteract immune resistance.
How Aging Shapes the Evolution of KRAS-Driven Lung Adenocarcinoma
Lazure et al. (2025). Aging directs the differential evolution of KRAS-driven lung adenocarcinoma. bioRxiv. https://doi.org/10.1101/2025.01.20.633951
The paper in one sentence:
Aging significantly influences the progression of KRAS-driven lung adenocarcinoma (LUAD), steering tumor evolution toward a primitive, stem-like state that worsens prognosis.
Summary:
Lung adenocarcinoma (LUAD), the most common type of lung cancer, disproportionately affects older individuals. This study investigates how aging alters the tumor microenvironment (TME) and drives distinct evolutionary trajectories of KRAS-mutant LUAD. Using a mouse model and single-cell RNA sequencing, the researchers demonstrate that tumors in older mice develop a more stem-like, treatment-resistant state. A key driver of this shift is the accumulation of damage-associated alveolar differentiation intermediate (ADI) cells, which disrupt normal lung homeostasis and enhance cancer stemness.
Personal highlights:
Aging Influences Tumor Evolution: LUAD tumors in older mice follow a distinct, non-canonical evolutionary path compared to those in younger mice.
Stemness and Poor Prognosis: Aged LUAD tumors acquire a primitive, stem-like identity associated with higher resistance to treatment.
Role of ADI Cells: A previously rare lung cell type, ADIs, accumulates in the aged tumor microenvironment, driving cancer evolution.
Why should we care?
Cancer research often focuses on genetic mutations, but this study underscores the crucial role of the body's aging environment in shaping cancer progression. With an aging global population, understanding how age-related biological changes influence cancer evolution is vital for developing better, more personalized treatments.
Digoxin as a Potential Game-Changer in Metastatic Breast Cancer Treatment
Kurzeder et al. (2025). Digoxin for reduction of circulating tumor cell cluster size in metastatic breast cancer: a proof-of-concept trial. Nature Medicine. https://doi.org/10.1038/s41591-024-03486-6
The paper in one sentence:
This proof-of-concept trial demonstrates that digoxin, a Na+/K+ ATPase inhibitor, can reduce the size of circulating tumor cell (CTC) clusters in metastatic breast cancer, potentially disrupting metastasis.
Summary:
Metastasis remains the leading cause of breast cancer mortality, with circulating tumor cell (CTC) clusters playing a crucial role in disease progression. This study tested whether digoxin, a well-known heart medication, could reduce CTC cluster size and disrupt their metastatic potential. In a small cohort of metastatic breast cancer patients, daily digoxin treatment for seven days led to a significant decrease in CTC cluster size. Transcriptomic analysis confirmed downregulation of genes involved in cell–cell adhesion and cell cycle progression. Importantly, the treatment was well tolerated with no adverse events. These findings suggest that repurposing digoxin—or refining Na+/K+ ATPase inhibitors—could offer a novel strategy for targeting metastasis in breast cancer.
Personal highlights:
Digoxin Reduces CTC Cluster Size: A 7-day treatment with digoxin significantly decreased the average number of cells per CTC cluster (mean reduction: 2.2 cells, P = 0.003).
Mechanistic Insights: Gene expression analysis showed suppression of cell–cell adhesion and cell cycle genes, supporting digoxin’s cluster-dissolution effect.
Well-Tolerated Treatment: No adverse effects were reported, indicating that digoxin at therapeutic doses is safe for cancer patients.
Why should we care?
Current cancer treatments primarily focus on shrinking primary tumors, often overlooking the mechanisms of metastasis. This study introduces a paradigm shift—by disrupting CTC clusters, we may be able to prevent the formation of secondary tumors. With metastasis being the leading cause of cancer-related deaths, targeting this process directly could significantly improve patient outcomes. Moreover, repurposing an existing, widely available drug like digoxin accelerates the path to clinical implementation.
How Cancer Cells Evade Pyroptosis to Boost Metastasis
Verma et al. (2025). Avoidance of pyroptosis accounts for the relatively high metastatic potential observed in early hybrid EMT states. bioRxiv. https://doi.org/10.1101/2025.01.26.634904
The paper in one sentence:
Cancer cells in early hybrid epithelial-mesenchymal transition (EMT) states evade pyroptosis—a form of inflammatory cell death—by downregulating ARRDC4 and Gasdermin E (GSDME), allowing them to metastasize more efficiently.
Summary:
This study explores how early hybrid EMT states—cells that exhibit both epithelial and mesenchymal characteristics—achieve higher metastatic potential. The researchers identified ARRDC4 as a key suppressor of metastasis. High ARRDC4 levels promote pyroptosis, an inflammatory form of programmed cell death triggered by Gasdermin E. However, cancer cells downregulate ARRDC4, ensuring sustained glucose uptake and protection from immune attack. This allows them to survive in circulation and seed metastases. Clinical data further reveal that ARRDC4 and GSDME levels are lower in aggressive breast cancer cases, correlating with worse patient outcomes.
Personal highlights:
Hybrid EMT Cells Are Highly Metastatic: Cancer cells in early EMT states have the highest ability to invade and spread.
Avoiding Pyroptosis Aids Survival: Metastatic cancer cells evade pyroptosis, a form of immune-mediated cell death, by suppressing ARRDC4 and GSDME.
Glucose Metabolism Fuels Tumor Spread: ARRDC4 downregulation allows cells to maintain glucose uptake, supporting their energy needs for metastasis.
Immune System Evasion: Tumors lacking ARRDC4 are better at escaping immune surveillance, especially from natural killer (NK) cells.
Clinical Relevance: Low ARRDC4 and GSDME expression in breast cancer patients predicts poorer survival, making them potential therapeutic targets.
Why should we care?
Understanding how cancer cells evade programmed cell death is crucial for developing new treatments. This study reveals that targeting the ARRDC4-GSDME pathway could restore the immune system’s ability to eliminate circulating tumor cells, preventing metastasis before it starts. With metastasis being the primary cause of cancer deaths, these findings offer a potential breakthrough in designing therapies to stop cancer spread.
Other papers that peeked my interest and were added to the purgatory of my “to read” pile
scGraphETM: Graph-Based Deep Learning Approach for Unraveling Cell Type-Specific Gene Regulatory Networks from Single-Cell Multi-Omics Data: https://www.biorxiv.org/content/10.1101/2025.01.24.634773v1
Evaluation of out-of-distribution detection methods for data shifts in single-cell transcriptomics: https://www.biorxiv.org/content/10.1101/2025.01.24.634709v1
Building Foundation Models to Characterize Cellular Interactions via Geometric Self-Supervised Learning on Spatial Genomics: https://www.biorxiv.org/content/10.1101/2025.01.25.634867v1
A genome-wide atlas of human cell morphology: https://www.nature.com/articles/s41592-024-02537-7
Refining the cis-regulatory grammar learned by sequence-to-activity models by increasing model resolution: https://www.biorxiv.org/content/10.1101/2025.01.24.634804v1
Categorization of 34 computational methods to detect spatially variable genes from spatially resolved transcriptomics data: https://www.nature.com/articles/s41467-025-56080-w
Thanks for reading.
Cheers,
Seb.


